Abstract
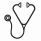
Background: Patients with FLT3-mutation-positive (FLT3mut+) acute myeloid leukemia (AML) have a poor prognosis, particularly those with a high allelic burden of FLT3-ITD mutations (FLT3-ITD mut+). Further, patients with FLT3-ITD mut+ who have relapsed after allogeneic hematopoietic stem cell transplantation (HSCT) have a 1-year overall survival (OS) rate of less than 20%. While treatment guidelines vary in their recommendations for maintenance therapy after HSCT to prevent relapse, data are emerging on the potential benefits of maintenance therapy for patients with FLT3mut+ AML.
Aim/Objective: To examine real-world survival outcomes in adult patients with FLT3-ITD mut+ AML who received maintenance therapy versus those who did not receive maintenance therapy after allogeneic HSCT, including a qualitative comparison with real-world survival outcomes in adults with FLT3mut+ AML.
Methods: This was a retrospective chart review wherein hematologists and oncologists from North America, Europe, and Japan extracted data from the medical charts of patients with FLT3mut+ AML who underwent HSCT after achieving complete remission with first-line chemotherapy within the prior 3 years. The index date was the date of HSCT and the study period was from the index date to the date of the last follow-up or death. All patients were grouped into two cohorts based on post-HSCT therapy received (no maintenance therapy or maintenance therapy). In an analysis of a subgroup of patients typically considered to be at high risk of relapse, patients who had both received an allogeneic HSCT and had a high allelic burden of FLT3-ITD mut+ were analyzed; patients with FLT3-ITD and FLT3-TKD co-mutations were also included in this subgroup. Overall survival during the study period was assessed for each cohort in the overall population of patients and in the subgroup. Kaplan-Meier analyses and Cox regression models, including unadjusted models and models with adjustments for baseline covariates, were used to describe and evaluate cross-cohort comparisons of survival. Covariates in the adjusted Cox models were Eastern Cooperative Oncology Group status, risk status, measurable residual disease status, age at index date, sex, extramedullary involvement, race, BMI, time from diagnosis to index month, HSCT type, and country.
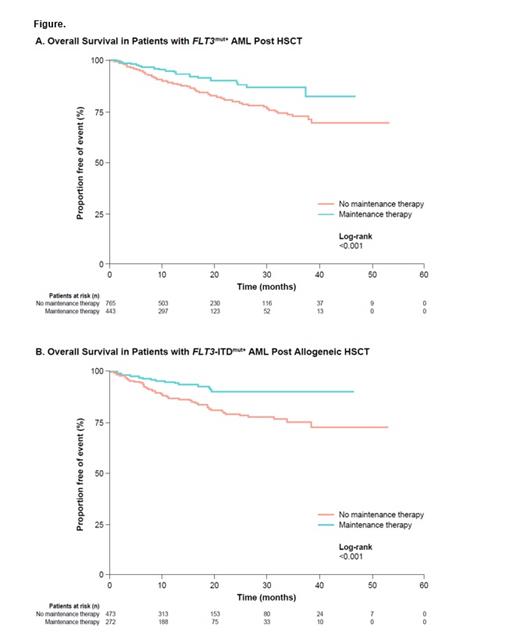
Results: A total of 1,208 AML patients with FLT3mut+ who received HSCT were included in the general study population; 765 (63.3%) patients received no maintenance therapy and 443 (36.7%) patients received maintenance therapy (including FLT3 inhibitors, hypomethylating agents, cytotoxic chemotherapy, and other targeted therapies). In Kaplan-Meier analyses, OS was longer in patients who received maintenance therapy compared with those who did not receive maintenance therapy (log-rank P<0.001; Figure A). Similar results were seen between maintenance therapy versus no maintenance therapy in an unadjusted Cox regression model (HR 0.52 [95% CI 0.35, 0.76], P<0.001) and adjusted Cox regression model (HR 0.48 [95% CI 0.30, 0.77], P<0.01).
In an analysis of the subgroup, data from the charts of 745 patients with FLT3-ITD mut+ who received allogeneic HSCT were reviewed. The mean age at HSCT was 53.2 years; 39.9% and 38.4% of patients had intermediate and poor risk status, respectively. Of this subgroup, 473 (63.5%) patients received no maintenance therapy and 272 (36.5%) patients received maintenance therapy. Kaplan-Meier analyses show that OS was longer in patients receiving maintenance therapy versus no maintenance therapy (log-rank P<0.001; Figure B); the risk of death appeared to plateau after approximately 2 years in patients receiving maintenance treatments. Similar results were seen between maintenance therapy versus no maintenance therapy in an unadjusted Cox regression model (HR 0.39 [95% CI 0.23, 0.65], P<0.001) and adjusted Cox regression model (HR 0.38 [95% CI 0.20, 0.72], P<0.01).
Conclusions: In patients with FLT3-ITD mut+ AML, OS was improved in patients that received any type of maintenance therapy compared with patients that received no maintenance therapy after allogeneic HSCT. These improved clinical outcomes in a high-risk subgroup receiving maintenance treatments are consistent with findings in the general population of patients with FLT3mut+ AML. Additional analyses are warranted to statistically verify these results.
Yang: Astellas Pharma, Inc.: Consultancy. Song: Astellas Pharma, Inc.: Consultancy. Griffin: Astellas Pharma, Inc.: Consultancy; Novartis: Patents & Royalties: Post marketing royalties from midostaurin. Shah: Astellas Pharma, Inc.: Current Employment; University of Michigan School of Public Health Department of Health Management and Policy Alumni Board: Other: Chair-Elect. Freimark: Astellas Pharma, Inc.: Consultancy. Chilelli: Astellas Pharma, Inc.: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract